PRE-CLINICAL STUDIES (in vitro)
3 in vitro studies viz.,(1)Effect on Intra cellular Lipid accumulation & Cholesterol Synthesis using Human Hepatocytes Cell Line using murine – in vitro;(2) effect on HMGCoA Reductase enzyme Activity – in vitro and (3) effect on pancreatic Lipase activity Activity-in vitro.[Dr manu Jaggi et al., althea Life sciences ,Sahibad 2015]
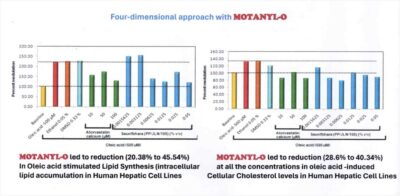
MOTANYL-O led to reduction (20.38% to 45.54%) In Oleic acid stimulated Lipid Synthesis(intracellular lipid accumulation in Human Hepatic Cell Lines
MOTANYL-O led to reduction (2806to40.34%) at all concentrations in oleic acid- induced Cellular Cholesterol levels in Human Hepatic Cell Lines
MOTANYLO led to inhibition (38.24%) pf HMG CoA Reductase Enzyme Activity at all concentrations
MOATANYL-O led to inhibition (50.7%) of Pancreatic Lipase enzyme Activity at all concentrations.
#HMG-CoA (3-hydroxy-3methylglutaryl coenzyme A reductase ) is the rate controlling enzyme of the mevalonate pathway (the metabolic pathway that produces cholesterol & other isoprenoids).
PRE – CLINICAL STUDIES(in vivo)
An experimental study to Evaluate Hypolipidemic and Anti-obesity Property of JLN/PP/105/ in High Fat Diet induced rat model [Dr.S.Venkatraman et al., Baid Metha Foundation for Educ.in pharm.Res.Chennai,2011]
Oral administration of PP/JLN/105(8 &16 mg/kg/p.o) showed significant (p<0.01,p<0.05) decrease in body weight as compared with group II animals.
Group II fed HFD exhibited significant (p<0.01) increase in total cholesterol when compared with group I animals. Group III to Group V animals exhibited a significant (p<0.01) decrease total cholesterol when compared with group II animals.
Group II animals when compared with group I animals exhibited significant (p<0.01) increase in LDL levels. Groups III and IV exhibited significant (p<0.05) decrease when compared to group II animals. Group V animals exhibited significant (p<0.01) decrease LDL levels.
MOTANYL-O (PP/JLN/105) at two dose levels 8mg/kg and 16mg/kg and Sibutamine 5mg/kg body weight were administered orally for 40 days to the HFD fed rats. It significantly reduced body weight , feed intake, lipid profile(TC,TGL,LDL and VLDL) and increased body temperature and HDL level.
In HFD induced obesity ,MOTANYL-O(PP/JLN/105) at 8mg/kg and 16mg/kg and Sibutramine 5mg/kg exhibited significant decrease in the blood sugar and total protein.
From the observations of the study performed, it could be predicted that MOTANYL-O (PP/JLN/105) exerted significant anti- obese activity due to its hypophagic , hypoglycaemic and hypolipidemic effect in rats fed on high fat diet. CLINICAL STUDIES
A Three Arm, Randomized, Open Label, Active-Controlled, Clinical Study to Evaluate Efficacy and Safety of
MOTANYL (PP/JLN/105) in the Management of Dyslipidemia &Body Weight
(CTRL Registration No: CTRI/2016/01/006494 dated 07.01.2016 & Part II, 21CFR compliant (e CRF based) clinical study)
(Study Duration: 12Weeks; N=95) Gr A-31, Gr B-34, and Gr C-30 with 1cap b.i.d., 2cap b.i.d and guggulu 1 b.i.d. respectively)
A significant (p<0.05) reduction was observed in LDL-C, Total Cholesterol, both Waist and Hip circumference, Total Cholesterol: HDL ratio, LDL: HDL ratio and quality of life measured through EQ-5D in all the three study groups at the end of study when compared with baseline. Comparison between the groups were not significant.
NOTE: The preclinical studies are indicative of the fact that three is a statistically significant reduction in the serum cholesterol as well as body weight following the administration of MOTANYL – O. The clinical study is suggestive of the fact that three is a reduction of 1 to 2.5kg reduction in the body weight and corresponding reduction in the BMI (which is statistically not significant). Further treatment with MOTANYL-O will provide significant results.
CLINICAL STUDIES
A Three Arm, Randomized, Open Label, Active-Controlled, Clinical Study to Evaluate Efficacy and Safety of
MOTANYL (PP/JLN/105) in the Management of Dyslipidemia &Body Weight
(CTRL Registration No: CTRI/2016/01/006494 dated 07.01.2016 & Part II, 21CFR compliant (e CRF based) clinical study)
(Study Duration: 12Weeks; N=95) Gr A-31, Gr B-34, and Gr C-30 with 1cap b.i.d., 2cap b.i.d and guggulu 1 b.i.d. respectively)
A significant (p<0.05) reduction was observed in LDL-C, Total Cholesterol, both Waist and Hip circumference, Total Cholesterol: HDL ratio, LDL: HDL ratio and quality of life measured through EQ-5D in all the three study groups at the end of study when compared with baseline. Comparison between the groups were not significant.
NOTE: The preclinical studies are indicative of the fact that three is a statistically significant reduction in the serum cholesterol as well as body weight following the administration of MOTANYL – O. The clinical study is suggestive of the fact that three is a reduction of 1 to 2.5kg reduction in the body weight and corresponding reduction in the BMI (which is statistically not significant). Further treatment with MOTANYL-O will provide significant results.




Reviews
There are no reviews yet.